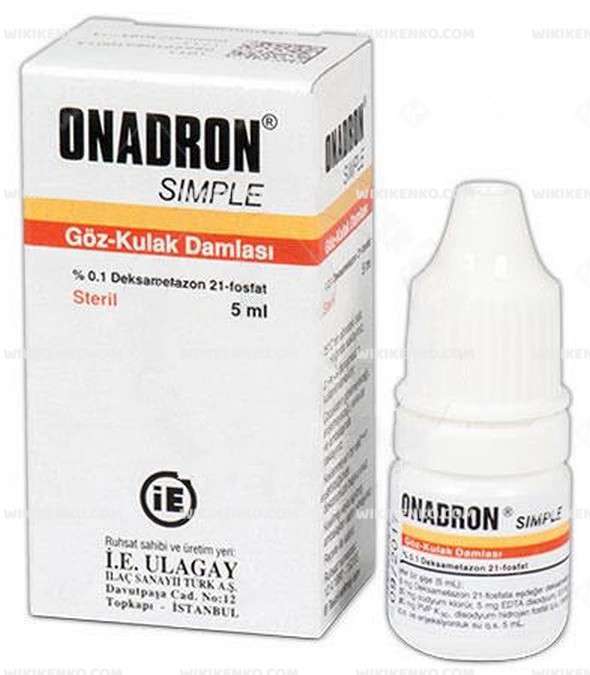
Onadron Simple Eye/Ear Drops is a safe and effective medicine for treating short-term inflammatory eye conditions and ear infections. If you’re experiencing redness, itching, or irritation in your eyes or ears, this medicine can provide you with fast relief. Just remember to always discuss any concerns you have with your doctor or pharmacist and to only use this medicine as directed.
| Potency |
%0.1 5Ml |
|---|---|
| Manufacturer |
I.E. Ulagay |
| Origin |
Turkey |
| Generic Name (Ingredient) |
Dexamethasone 21-Phosphate Disodium (5 Mg Dexamethasone 21-Phosphate Equivalent) 5.63 Mg |
Assuming your emergency circumstances for this product, visit Urgent Quotation page. Besides, for any pharmaceutical questions, please ask us in the comments section.
-
Description
-
Comments
-
Report / Upload
-
Disclaimer
Description
Usage
Onadron Simple Eye/Ear Drops are primarily used to treat short-term inflammatory eye conditions. If you’re experiencing redness, itching, or irritation in your eyes, this medicine can provide you with fast relief. Dexamethasone, the active ingredient in Onadron, works by reducing the inflammation in your eyes and decreasing the amount of swelling and redness you’re experiencing.
In addition to treating eye conditions, Onadron Simple Eye/Ear Drops can also be used to treat ear infections. If you’re experiencing ear pain, discharge, or swelling, Onadron can help relieve these symptoms and get you back to feeling your best.
Side Effects
As with all medicines, there is a risk of side effects when using Onadron Simple Eye/Ear Drops. However, there is limited information available on the specific side effects of this medicine. It’s important to note that all medicines have the potential to cause side effects, so it’s always a good idea to discuss any concerns you have with your doctor or pharmacist.
Availability
Onadron Simple Eye/Ear Drops are manufactured by I.E. Ulagay and originate from Turkey. While availability may be limited outside of Turkey, you may still be able to purchase this medicine through online pharmacies or by speaking with your doctor or pharmacist.
Conclusion
Onadron Simple Eye/Ear Drops active ingredient is Dexamethasone 21-Phosphate Disodium has been proven to provide fast relief from redness, itching, and irritation in the eyes and ears. While there may be limited availability of this medicine outside of Turkey, it may still be possible to purchase it through online pharmacies or with the guidance of your doctor or pharmacist. As with any medicine, it’s important to use Onadron Simple Eye/Ear Drops as directed and to discuss any concerns with your healthcare provider.
Use the form below to report an error
Please answer the questions as thoroughly and accurately as possible. Your answers will help us better understand what kind of mistakes happen, why and where they happen, and in the end the purpose is to build a better archive to guide researchers and professionals around the world.
The information on this page is not intended to be a substitute for professional medical advice, diagnosis, or treatment. always seek the advice for your physician or another qualified health provider with any questions you may have regarding a medical condition. Always remember to
- Ask your own doctor for medical advice.
- Names, brands, and dosage may differ between countries.
- When not feeling well, or experiencing side effects always contact your own doctor.
Cyberchondria
The truth is that when we’re sick, or worried about getting sick, the internet won’t help.
According to Wikipedia, cyberchondria is a mental disorder consisting in the desire to independently make a diagnosis based on the symptoms of diseases described on Internet sites.
Why you can’t look for symptoms on the Internet
If diagnoses could be made simply from a textbook or an article on a website, we would all be doctors and treat ourselves. Nothing can replace the experience and knowledge of specially trained people. As in any field, in medicine there are unscrupulous specialists, differences of opinion, inaccurate diagnoses and incorrect test results.
People also search for…
-
-
-
Onadron Ampul
-
Rated 5.00 out of 5
-
Дексаметазон (Dexamethasone)
💊 Состав препарата Дексаметазон
✅ Применение препарата Дексаметазон
Описание активных компонентов препарата
Дексаметазон
(Dexamethasone)
Приведенная научная информация является обобщающей и не может быть использована для принятия
решения о возможности применения конкретного лекарственного препарата.
Дата обновления: 2022.05.27
Владелец регистрационного удостоверения:
Код ATX:
S01BA01
(Дексаметазон)
Лекарственная форма
| Дексаметазон |
Капли глазные 0.1%: фл.-капельн. 10 мл 1 шт. рег. №: ЛСР-001806/08 |
Форма выпуска, упаковка и состав
препарата Дексаметазон
Капли глазные 0.1% в виде суспензии белого цвета; возможно присутствие осадка, который быстро ресуспендируется при легком взбалтывании.
Вспомогательные вещества: полисорбат 80, гипромеллоза (гидроксипропилметилцеллюлоза 4000), динатрия фосфат додекагидрат, лимонной кислоты моногидрат, натрия хлорид, динатрия эдетат дигидрат, бензалкония хлорид, вода очищенная.
10 мл — флакон-капельницы полимерные (1) — пачки картонные.
Фармакологическое действие
ГКС. Подавляет функции лейкоцитов и тканевых макрофагов. Ограничивает миграцию лейкоцитов в область воспаления. Нарушает способность макрофагов к фагоцитозу, а также к образованию интерлейкина-1. Способствует стабилизации лизосомальных мембран, снижая тем самым концентрацию протеолитических ферментов в области воспаления. Уменьшает проницаемость капилляров, обусловленную высвобождением гистамина. Подавляет активность фибробластов и образование коллагена.
Ингибирует активность фосфолипазы А2, что приводит к подавлению синтеза простагландинов и лейкотриенов. Подавляет высвобождение ЦОГ (главным образом ЦОГ-2), что также способствует уменьшению выработки простагландинов.
Уменьшает число циркулирующих лимфоцитов (T- и B-клеток), моноцитов, эозинофилов и базофилов вследствие их перемещения из сосудистого русла в лимфоидную ткань; подавляет образование антител.
Дексаметазон подавляет высвобождение гипофизом АКТГ и β-липотропина, но не снижает уровень циркулирующего β-эндорфина. Угнетает секрецию ТТГ и ФСГ.
При непосредственной аппликации на сосуды оказывает вазоконстрикторный эффект.
Дексаметазон обладает выраженным дозозависимым действием на метаболизм углеводов, белков и жиров. Стимулирует глюконеогенез, способствует захвату аминокислот печенью и почками, повышает активность ферментов глюконеогенеза. В печени дексаметазон усиливает депонирование гликогена, стимулируя активность гликогенсинтетазы и синтез глюкозы из продуктов белкового обмена. Повышение содержания глюкозы в крови активизирует выделение инсулина.
Дексаметазон подавляет захват глюкозы жировыми клетками, что приводит к активации липолиза. Однако вследствие увеличения секреции инсулина происходит стимуляция липогенеза, что приводит к накоплению жира.
Оказывает катаболическое действие в лимфоидной и соединительной ткани, мышцах, жировой ткани, коже, костной ткани. Остеопороз и синдром Иценко-Кушинга являются главными факторами, ограничивающими длительную терапию ГКС. В результате катаболического действия возможно подавление роста у детей.
В высоких дозах дексаметазон может повышать возбудимость тканей мозга и способствует понижению порога судорожной готовности. Стимулирует избыточную продукцию хлористоводородной кислоты и пепсина в желудке, что способствует развитию пептической язвы.
При местном применении терапевтическая активность дексаметазона обусловлена противовоспалительным, противоаллергическим и антиэкссудативным (благодаря вазоконстрикторному эффекту) действием.
Фармакокинетика
При местном применении в офтальмологии всасывается через роговицу с интактным эпителием во влагу передней камеры глаза. При воспалении тканей глаза или повреждении слизистой оболочки и роговицы скорость всасывания дексаметазона достоверно увеличивается.
Показания активных веществ препарата
Дексаметазон
Аллергические и воспалительные заболевания глаз: негнойный и аллергический конъюнктивит, кератит, кератоконъюнктивит без повреждения эпителия, ирит, иридоциклит, блефароконъюнктивит, блефарит, эписклерит, склерит, воспалительный процесс после травм глаза и оперативных вмешательств, симпатическая офтальмия; макулярный отек вследствие окклюзии центральной вены сетчатки или ее ветвей, нарушения зрения вследствие диабетического макулярного отека у пациентов с артифакией, у пациентов, имеющих недостаточный ответ на терапию, или тех, кому не подходит терапия препаратами, отличными от ГКС, воспаление сосудистой оболочки заднего отдела глаза, представляющее собой неинфекционный увеит.
Режим дозирования
Способ применения и режим дозирования конкретного препарата зависят от его формы выпуска и других факторов. Оптимальный режим дозирования определяет врач. Следует строго соблюдать соответствие используемой лекарственной формы конкретного препарата показаниям к применению и режиму дозирования.
В офтальмологии способ применения зависит от используемой лекарственной формы. Схема лечения зависит от показаний к применению.
Побочное действие
Со стороны нервной системы: нечасто — дисгевзия.
Со стороны органа зрения: часто — дискомфорт в глазах; нечасто — кератит, конъюнктивит, синдром «сухого глаза», окрашивание роговицы медицинским красителем, фотофобия, затуманивание зрения, зуд в глазу, чувство инородного тела в глазах, повышенное слезоотделение, необычные ощущения в глазу, образование корок на краях век, раздражение глаз, гиперемия глаз.
Со стороны иммунной системы: гиперчувствительность.
Со стороны эндокринной системы: синдром Иценко-Кушинга, надпочечниковая недостаточность.
Со стороны нервной системы: головокружение, головная боль.
Со стороны органа зрения: глаукома, язвенный кератит, повышение внутриглазного давления, снижение остроты зрения, эрозия роговицы, птоз век, боль в глазу, мидриаз.
Противопоказания к применению
Для кратковременного применения по жизненным показаниям — повышенная чувствительность к дексаметазону.
Бактериальные, вирусные, грибковые заболевания глаз, туберкулезное поражение глаз, нарушение целостности глазного эпителия, острая форма гнойной глазной инфекции при отсутствии специфической терапии, заболевания роговицы, сочетающиеся с дефектами эпителия, трахома, глаукома с декомпенсацией повышения внутриглазного давления, не купирующаяся лекарственными препаратами, афакия с разрывом задней капсулы хрусталика; возраст до 18 лет (для некоторых лекарственных форм).
Применение при беременности и кормлении грудью
При беременности (особенно в I триместре), а также в период лактации дексаметазон применяют с учетом ожидаемого лечебного эффекта и отрицательного влияния на плод. При длительной терапии при беременности не исключена возможность нарушений роста плода. В случае применения в конце беременности существует опасность возникновения атрофии коры надпочечников у плода, что может потребовать проведения заместительной терапии у новорожденного.
Применение при нарушениях функции печени
С осторожностью следует применять при тяжелой хронической печеночной недостаточности.
Применение при нарушениях функции почек
С осторожностью следует применять при тяжелой хронической почечной недостаточности.
Применение у детей
Не рекомендуется применять у детей.
Противопоказание: возраст до 18 лет (для некоторых лекарственных форм).
Применение у пожилых пациентов
С осторожностью применять у пациентов пожилого возраста во избежание риска обострения хронических заболеваний.
Особые указания
Длительное применение местных ГКС может приводить к повышению внутриглазного давления и/или глаукоме с поражением зрительного нерва, к снижению остроты зрения и дефектам полей зрения, к образованию задней субкапсулярной катаракты. Поэтому у пациентов длительное время (более 10 дней) применяющих препараты, содержащие ГКС, следует регулярно измерять внутриглазное давление.
Риск повышения внутриглазного давления и/или образования катаракты вследствие применения кортикостероидов у пациентов с предрасположенностью (например, с сахарным диабетом) более высок. Риск повышения внутриглазного давления увеличивается у пациентов с сопутствующими офтальмогипертензией и/или глаукомой, а также у пациентов с семейным анамнезом глаукомы. Необходим еженедельный контроль внутриглазного давления у таких пациентов.
Следует соблюдать осторожность и периодически проводить биомикроскопию при применении препарата для терапии глубоких кератитов, вызванных Herpes simplex.
При отсутствии улучшений в течение 7-8 дней необходимо пересмотреть выбор терапии.
ГКС способны снижать устойчивость к бактериальным, вирусным, грибковым или паразитарным инфекциям и способствовать их развитию, а также маскировать клинические признаки инфекции.
При сопутствующих бактериальных инфекциях должна быть назначена соответствующая антибактериальная терапия.
Появление на роговице незаживающих язв может свидетельствовать о развитии грибковой инвазии. При возникновении грибковой инвазии терапию ГКС необходимо прекратить.
ГКС при местном применении могут замедлять процесс заживления роговицы.
Известно, что при заболеваниях, которые вызывают истончение роговицы или склеры, могут возникать перфорации в результате использования ГКС для местного применения.
При длительности терапии более 2 недель следует контролировать состояние роговицы. Применение дексаметазона в комплексной терапии синдрома Шегрена возможно только при кератоконъюнктивите средней и тяжелой степени тяжести, длительность курса терапии должна составлять не более 2 недель ввиду возможности развития нежелательных реакций.
После применения рекомендуется произвести носослезную окклюзию или осторожно закрыть глаз. Это может снизить системную абсорбцию препарата при местном применении, и тем самым уменьшить вероятность возникновения системных нежелательных реакций
При лечении воспаления глаз контактные линзы носить не рекомендуется.
Местное и системное применение ГКС может приводить к зрительным нарушениям. Если у пациента развиваются такие симптомы, как помутнение зрения или другие нарушения зрения, он должен быть направлен к офтальмологу для выявления возможных причин их развития, среди которых могут быть катаракта, глаукома или редкие заболевания, такие как центральная серозная хориоретинопатия, о которых сообщалось после использования системных и местных ГКС.
ГКС для местного применения не следует применять до установления этиологии поражения глаз.
Влияние на способность к управлению транспортными средствами и механизмами
Временное снижение четкости зрения или другие нарушения зрения могут влиять на способность к вождению автотранспорта или управлению механизмами. Если у пациента после применения препарата временно снижается четкость зрения, то перед вождением автотранспорта или управлением механизмами ему следует подождать до восстановления зрения.
Лекарственное взаимодействие
При одновременном применении с антипсихотическими средствами, букарбаном, азатиоприном возникает риск развития катаракты; со средствами, оказывающими антихолинергическое действие — риск развития глаукомы.
Одновременное применение местных стероидов и НПВС для местного применения может усиливать вероятность нарушений заживления роговицы.
Имеются данные о возникновении геморрагических осложнений, включая субконъюнктивальное кровоизлияние при применении дексаметазона в офтальмологии в некоторых лекарственных формах у пациентов, получающих антиагреганты и антикоагулянты. При необходимости одновременного применения требуется осторожность.
Нельзя исключить риск дополнительного повышения внутриглазного давления, если дексаметазон применяется совместно с антихолинергическими средствами, которые также могут вызвать повышение внутриглазного давления у предрасположенных пациентов. В случае совместного применения с противоглаукомными препаратами возможно снижение гипотензивного эффекта последних.
Ингибиторы CYP3A4, включая ритонавир и кобицистат, способны повышать уровень системного воздействия, что приводит к увеличению риска развития угнетения функции надпочечников/синдрома Кушинга. Следует избегать комбинирования данных препаратов, за исключением тех случаев, когда благоприятное действие превышает повышенный риск развития системных побочных эффектов ГКС, но в этом случае пациент должен находиться под тщательным наблюдением на предмет возникновения системных эффектов кортикостероидов.
В случае применения с другими местными офтальмологическими препаратами интервал между их применением должен составлять не менее 10 мин.
Глазные мази следует применять в последнюю очередь.
Если вы хотите разместить ссылку на описание этого препарата — используйте данный код
Спасатели Что такое Onadron Simple и что он делает? автор: Admin, 24 июля 2020 г.
Content
Что такое Onadron Simple и для чего он нужен?
онадрон простой содержит 0,1% дексаметазона. Применяется в виде глазно-ушных капель в пластиковых флакончиках по 5 мл с дозатором. Хорошо помогает при аллергическом конъюнктивите и воспалении наружного уха. Это продукт фармацевтической компании e ulagay.
Сколько стоят ушные капли?
ИНФОРМАЦИЯ О ПРЕПАРАТАХ
Код погашения A07319 Цена продажи 17,12 турецких лир [ 25 апреля 2022 г. ] Предыдущая цена продажи 17,12 турецких лир [ 18 апреля 2022 г. ] Статус рецепта оригинального / непатентованного непатентованного лекарства Это обычный рецептурный препарат. Что такое Моксидекса?
Местное лечение бактериальных инфекций, вызванных штаммами, чувствительными к моксифлоксацину, в переднем сегменте глаза, требующее комбинации кортикостероидов и антибиотиков • Чувствительные к стероидам воспалительные заболевания с риском поверхностной бактериальной инфекции или бактериальной глазной инфекции, при которых показаны кортикостероиды. …
Ушные капли Ципрогут не соответствуют возрастным ограничениям?
СИПРОГУТ не следует применять у детей младше 1 года. Применение у пожилых людей: коррекции дозировки у пожилых людей не требуется.
Удаляет ли оксибор ушную серу?
Врачи рекомендуют не использовать белые ушные палочки при применении этого препарата. Если и это лекарство не действует, врач чистит ухо сам.
Как удалить ушную серу?
В качестве первого метода раствор можно распылять до получения прозрачного цвета и повторять процесс промывки. В качестве второго раствора, после нанесения раствора и его размягчения, он может осторожно очистить ушную серу кюреткой. Или ваш врач может пропылесосить пробку с помощью аспиратора после нанесения раствора.
Сколько минут должны оставаться ушные капли?
Если вы используете другие ушные капли; Вы должны оставить 10-15-минутный интервал между последовательными закапываниями.
Должны ли ушные капли попасть в ухо?
Использование ушных капель: закапывайте ушные капли, оттягивая ухо вверх и назад, оттягивая ушную раковину назад и вперед, чтобы позволить каплям попасть в слуховой проход. После этого процесса поднимите голову и подождите, пока вода из уха не вытечет.
Читать: 211
Страна: Турция
Язык: турецкий
Источник: TİTCK (Türkİye İlaç Ve Tibbİ Cİhaz Kurumu)
Купи это сейчас
Активный ингредиент:
deksametazon
Доступна с:
MENARİNİ SAĞLIK VE İLAÇ SAN. VE TİC. A.Ş.
код АТС:
S03BA01
ИНН (Международная Имя):
dexamethasone
Тип рецепта:
Normal
Терапевтические области:
deksametazon
Статус Авторизация:
Aktif
Дата Авторизация:
1990-04-12
тонкая брошюра
1/8
KULLANMA TALİMATI
ONADRON
® SIMPLE %0.1 GÖZ/KULAK DAMLASI
GÖZE/KULAĞA DAMLATILARAK UYGULANIR.
STERIL
•
_ETKIN MADDE: _
5 mg deksametazon 21-fosfata eşdeğer 5,466 mg deksametazon 21-fosfat
disodyum
içerir.
•
_YARDIMCI _
_MADDELER: _
Sodyum
klorür,
EDTA
disodyum,
benzalkonyum
klorür,
polivinil
prolidon
K-30,
disodyum
hidrojen
fosfat
heptahidrat,
mono
sodyum
hidrojen
fosfat
dihidrat, enjeksiyonluk su.
BU
ILACI
KULLANMAYA
BAŞLAMADAN
ÖNCE
BU
KULLANMA
TALİMATINI
DIKKATLICE
OKUYUNUZ,
ÇÜNKÜ SIZIN IÇIN ÖNEMLI BILGILER IÇERMEKTEDIR.
•
_ Bu kullanma talimatını saklayınız. Daha sonra tekrar okumaya
ihtiyaç duyabilirsiniz. _
•
_ Eğer ilave sorularınız olursa, lütfen doktorunuza veya
eczacınıza danışınız. _
•
_ Bu ilaç kişisel olarak sizin için reçete edilmiştir,
başkalarına vermeyiniz. _
•
_ Bu ilacın kullanımı sırasında, doktora veya hastaneye
gittiğinizde doktorunuza bu ilacı _
_kullandığınızı söyleyiniz. _
•
_Bu talimatta yazılanlara aynen uyunuz. İlaç hakkında size
önerilen dozun dışında YÜKSEK _
_VEYA DÜŞÜK doz kullanmayınız. _
_ _
BU KULLANMA TALIMATINDA:
_1._
_ _
_ONADRON_
_®_
_ SIMPLE NEDIR VE NE IÇIN KULLANILIR?_
_2._
_ _
_ONADRON_
_®_
_ SIMPLE’I KULLANMADAN ÖNCE DIKKAT EDILMESI GEREKENLER_
_3._
_ _
_ONADRON_
_®_
_ SIMPLE NASIL KULLANILIR?_
_4._
_ _
_OLASI YAN ETKILER NELERDIR?_
_5._
_ _
_ONADRON_
_®_
_ SIMPLE’IN SAKLANMASI_
_ _
BAŞLIKLARI YER ALMAKTADIR.
1.
ONADRON
® SIMPLE NEDIR VE NE IÇIN KULLANILIR?
ONADRON
®
SIMPLE
5ml’lik
beyaz
renkli
polietilen
damlalıklı
ve
kapaklı
beyaz
renkli
polipropilen şişelerde sunulan renksiz, berrak çözeltidir.
ONADRON
®
SIMPLE
gözün
ve
kulağın
infeksiyonlu
olmayan
dış
inflamatuvar
ve
alerjik
durumlarının
ve operasyon sonrası
inflamasyonun
tedavisinde
kullanılır.
ONADRON
®
SIMPLE, kortikosteroidler
olarak
adlandırılan
bir
grup
ilaçtan
biridir. Gözdeki
ve kulaktaki inflamasyonun
önlenmesine
veya azaltılmasına
yardımcı
olur.
Göz
yüzeyinin
ve
göz
içindeki
Прочитать полный документ
Характеристики продукта
1/9
KISA ÜRÜN BİLGİSİ
1.
BEŞERİ
TIBBİ
ÜRÜNÜN ADI
ONADRON
®
SIMPLE %0.1 göz/kulak damlası
2.
KALİTATİF
VE KANTİTATİF
BİLEŞİMİ
Her bir şişe (5 ml):
ETKIN MADDE:
Deksametazon 21-fosfat disodyum
5,63 mg
(5 mg deksametazon 21-fosfata eşdeğer)
YARDIMCI
MADDELER:
Benzalkonyüm klorür
0,5 mg
Yardımcı maddeler için 6.1’e bakınız.
3.
FARMASÖTİK FORMU
Göz-kulak
damlası.
Renksiz, berrak çözeltidir.
4.
KLİNİK ÖZELLİKLER
4.1. TERAPÖTIK ENDIKASYONLAR
Anteriyör
üveyit,
iritis,
siklitis,
alerjik
ve
vernal
konjunktivit,
herpes
zoster’in
neden
olduğu
keratit, yüzeysel punktat keratit ve spesifik olmayan yüzeysel
keratit gibi konjunktiva, kornea ve
gözün
ön segmentinin
steroide
cevap veren
inflamatuvar durumlarının tedavisinde endikedir.
Ayrıca
kimyasal,
radyasyon
veya
termal
yanıklardan
kaynaklanan
ya
da
yabancı
cisim
penetrasyonunu
takiben
meydana
gelen
korneal
hasarın
tedavisinde
endikedir.
İnflamatuvar
reaksiyonların
azaltılmasında
ve
graft
reaksiyonların
bastırılmasında
ameliyat
sonrası
kullanım
için
endikedir.
Allerjik
dış
kulak
iltihabı,
steroid
kullanımının
ödem
ve
inflamasyonunun
giderilmesi
için
gerekli
görüldüğü
pürülan
ve non pürülan
enfeksiyöz
dış kulak
iltihabı
tedavisinde
endikedir.
4.2. POZOLOJI VE UYGULAMA ŞEKLI
Oküler ve
kulak
içine kullanım
içindir.
POZOLOJI/UYGULAMA
SIKLIĞI VE SÜRESI:
Topikal olarak konjunktivaya bir veya iki damla uygulanır.
Ciddi
veya
akut
inflamasyonda,
tedavi
başlangıcında,
hasta
gözün/gözlerin
konjunktival
keselerine her 30-60 dakikada bir 1-2 damla
damlatılır.
2/9
Tedaviye
istenilen
cevap
alındıktan
sonra,
uygulamaların
sıklığı,
hasta
gözün/gözlerin
konjunktival keselerine her 2-4 saatte bir 1-2 damla olacak şekilde
azaltılmalıdır.
İnflamasyon
yeteri
kadar
kontrol
altına
alınıyorsa,
doz
günde
3-4
kere
bir
damlaya
kadar
azaltılabilir.
Eğer 3-4 gün içinde yeterli
cevap alınamazsa,
sistemik
veya subkonjonktival tedavi eklenebilir.
Kronik inflamasyonda, doz, hasta
Прочитать полный документ
Похожие продукты
Поиск оповещений, связанных с этим продуктом
Просмотр истории документов
- Скачать инструкцию медикамента
Торговое название
Вел Дроп
Международное непатентованное название
Нет
Лекарственная форма
Капли глазные
Состав
1 мл раствора содержит
активное вещество – натриевой соли кармеллозы** (натрия карбоксиметилцеллюлозы (Цекол 2000) 5,000 мг,
вспомогательные вещества: стабилизированный комплекс оксихлорида***, натрия хлорид, кальция хлорид дигидрат, калия хлорид, магния хлорида гексагидрат, кислота борная, кислота хлористоводородная 10 %, натрия гидроксид 10 %, вода для инъекций
Описание
Прозрачный бесцветный, слегка вязкий раствор.
Фармакотерапевтическая группа
Препараты для лечения заболеваний глаз прочие.
Код АТХ S01ХА
Фармакологические свойства
Фармакокинетика
Исследования фармакокинетики у человека не проводили. В исследованиях на животных данные о канцерогенности, мутагенности, влиянии на фертильность или тератогенных эффектах не были получены.
Фармакодинамика
Вел Дроп офтальмологический местный препарат. Компоненты средства не оказывают фармакологического действия, влияние препарата основано на физических свойствах веществ. Натрия карбоксиметилцеллюлоза оказывает увлажняющее действие и способствует стабилизации слезной пленки, используется в качестве смазывания сухих и раздраженных глаз. Основными причинами сухого глаза являются ветер, солнце, жара/ кондиционер, работа за компьютером/чтение и некоторые медикаменты. Глазные капли натрия карбоксиметилцеллюлозы сохраняют влажность глаз, защищают от повреждения и инфекции, и уменьшают симптомы сухих глаз, таких как жжение, зуд и чувство постороннего тела в глазу. Натрия карбоксиметилцеллюлоза из за высокого молекулярного веса, мало проникает в роговицу.
Показания к применению
— для временного облегчения жжения и раздражения глаз вследствие его сухости, а также в качестве защитного средства от дальнейшего раздражения
— для временного облегчения дискомфорта, вызванного небольшим раздражением глаз, воздействием на него ветра или солнца или при длительной работе на компьютере.
Способ применения и дозы
Закапывать по 1 или 2 капли в поврежденный глаз(а) по мере необходимости. Продолжительность лечения определяется лечащим врачом.
При совместном применении с другими местными офтальмологическими препаратами, необходимо соблюсти интервал между применением препаратов около 10-15 минут.
Не следует прикасаться кончиком пипетки к глазам, или любой другой поверхности, чтобы избежать загрязнения содержимого флакона.
Побочные действия
Местные
Часто:
-
нечеткость видения
-
дискомфорт глаза
-
астенопия
-
ощущение инородного тела в глазу
-
зуд глаз
-
гиперемия глаза
Не часто:
-
раздражение глаз
Частота проявления следующих побочных эффектов не может быть определена в связи с недостаточностью данных:
Местные: эритема века, отек глаза, боль в глазу, выделения из глаз, образование корок по краю век, увеличение слезоточивости.
Противопоказания
— повышенная чувствительность к любому компоненту препарата
— детский возраст до 18 лет
Лекарственные взаимодействия
Специфической оценки лекарственного взаимодействия для препарата Вел Дроп капли глазные не проводилось. Принимая во внимание состав препарата, проявление лекарственных взаимодействий маловероятно.
При применении более одного местного офтальмологического препарата, препараты должны применяться с интервалом не менее 5 минут.
Особые указания
Для местного применения в офтальмологии. Не для инъекций или приема внутрь.
При появлении во время использования препарата головной боли, боли в глазах, нарушения зрения, раздражения глаз, устойчивого покраснения или ухудшение перечисленных состояний, а также устойчивом их проявлении в течение более 3 дней, следует прекратить использование препарата и обратиться к врачу.
При использовании препарата у детей и подростков, а также у пациентов с почечной и печеночной недостаточностью необходимость регулирования дозировки отсутствует.
Беременность и период лактации
Компоненты препарата Декстран 70 и гипромеллоза оказывают влияние на защитные свойства поверхности глаза и не имеют фармакологической активности. Данные компоненты практически не проникают в системный кровоток. Препарат Вел Дроп капли глазные может применяться в период беременности/лактации в случае, если эффективность лечения превышает потенциальный риск для плода/грудного ребенка.
Особенности влияния лекарственного средства на способность управлять транспортным средством или потенциально опасными механизмами
Как и в случае с другими глазными каплями, после закапывания возможна временная неясность зрения или другие визуальные беспокойства, что может негативно повлиять на способность управлять автомобилем или другими потенциально опасными механизмами. В этом случае необходимо подождать
некоторое время до полного восстановления зрения.
Передозировка
Сообщений о случаях передозировки не получено.
При местном применении передозировка маловероятна.
Форма выпуска и упаковка
Капли глазные по 10 мл в пластиковой ампуле. По 1 ампуле вместе с инструкцией по медицинскому применению на государственном и русском языках помещают в картонную коробку.
Условия хранения
Хранить в оригинальной упаковке в защищенном от света месте при температуре не выше 25 °С.
Хранить в недоступном для детей месте!
Срок хранения
2 года
Не использовать после истечения срока хранения, указанного на упаковке.
Условия отпуска из аптек
Без рецепта
Производитель
Алембик Фармасьютикелс Лимитед, Индия
Владелец регистрационного удостоверения
Алембик Фармасьютикелс Лимитед, Индия
Адрес организации, принимающей на территории Республики Казахстан претензии от потребителей по качеству продукции
г. Алматы, ул. Кабанбай батыра 137, кв. 39
тел/факс +7(727) 292-72-75
Top 20 medicines with the same components:
Top 20 medicines with the same treatments:
Name of the medicinal product
The information provided in Name of the medicinal product of Onadron
is based on data of another medicine with exactly the same composition as the Onadron.
. Be careful and be sure to specify the information on the section Name of the medicinal product in the instructions to the drug Onadron directly from the package or from the pharmacist at the pharmacy.
more…
Onadron
Qualitative and quantitative composition
The information provided in Qualitative and quantitative composition of Onadron
is based on data of another medicine with exactly the same composition as the Onadron.
. Be careful and be sure to specify the information on the section Qualitative and quantitative composition in the instructions to the drug Onadron directly from the package or from the pharmacist at the pharmacy.
more…
Dexamethasone
Therapeutic indications
The information provided in Therapeutic indications of Onadron
is based on data of another medicine with exactly the same composition as the Onadron.
. Be careful and be sure to specify the information on the section Therapeutic indications in the instructions to the drug Onadron directly from the package or from the pharmacist at the pharmacy.
more…
Eye drops; Eye suspension; Injection; Pills; Solution for intravenous and intramuscular injection; Substance-powder
Coated tablet; Intravitreal implant in applicator; Имплантат для интравитреального введения
Eye ointment
Neurology
Cerebral oedema caused by brain tumours, neurosurgery, bacterial meningitis, brain abscess.
Pulmonary and respiratory diseases
Severe acute asthma attack.
Dermatology
Oral initial treatment of extensive, severe, acute skin diseases that respond to glucocorticoids, such as erythroderma, pemphigus vulgaris, acute eczema.
Autoimmune disorders/rheumatology
Oral initial treatment of autoimmune diseases, such as systemic lupus erythematosus (especially visceral forms).
Severely progressive form of active rheumatoid arthritis, e.g. rapidly destructive forms and/or with extra-articular manifestations.
Infectology
Severe infections with toxic conditions (e.g. tuberculosis, typhoid) only with concomitant anti-infective therapy.
Oncology
Palliative treatment of malignant tumours.
Endocrinology
Congenital adrenogenital syndrome in adulthood.
OZURDEX is indicated for the treatment of adult patients with:
— visual impairment due to diabetic macular oedema (DME) who are pseudophakic or who are considered insufficiently responsive to, or unsuitable for non-corticosteroid therapy
— macular oedema following either Branch Retinal Vein Occlusion (BRVO) or Central Retinal Vein Occlusion (CRVO)
— inflammation of the posterior segment of the eye presenting as non-infectious uveitis
Indicated for treatment of steroid responsive inflammatory conditions of the conjunctiva, cornea and anterior segment of the eye, such as, anterior uveitis, iritis, cyclitis, allergic and vernal conjunctivitis, herpes zoster keratitis, superficial punctate keratitis and non-specific superficial keratitis.
Also indicated for the treatment of corneal injury from chemical, radiation or thermal burns or following penetration by foreign bodies. Indicated for post-operative use to reduce inflammatory reactions and suppress graft reaction.
Dosage (Posology) and method of administration
The information provided in Dosage (Posology) and method of administration of Onadron
is based on data of another medicine with exactly the same composition as the Onadron.
. Be careful and be sure to specify the information on the section Dosage (Posology) and method of administration in the instructions to the drug Onadron directly from the package or from the pharmacist at the pharmacy.
more…
Eye drops; Eye suspension; Injection; Pills; Solution for intravenous and intramuscular injection; Substance-powder
Coated tablet; Intravitreal implant in applicator; Имплантат для интравитреального введения
Eye ointment
Posology
Dosage depends on the nature and severity of the disease and the individual response of the patient to treatment. In general, relatively high initial doses are administered, and they should be significantly higher in acute severe forms than in chronic diseases.
Unless otherwise prescribed, the following dosage recommendations apply:
— Cerebral oedema: Depending on the cause and severity, initial dose of 8-10 mg (up to 80 mg) i.v., followed by 16-24 mg (up to 48 mg)/day orally, divided into 3-4 (up to 6) individual doses for 4-8 days. A longer-term, lower-dose administration of Onadron Krka may be required during irradiation and in the conservative treatment of inoperable brain tumours.
— Cerebral oedema due to bacterial meningitis: 0.15 mg/kg body weight every 6 hours for 4 days, children: 0.4 mg/kg body weight every 12 hours for 2 days, starting before the first antibiotics.
— Severe acute asthma attack: Adults: 8-20 mg, then, if necessary, 8 mg every 4 hours. Children: 0.15-0.3 mg/kg body weight.
— Acute skin diseases: Depending on the nature and extent of the disease, daily doses of 8-40 mg. Followed by treatment with decreasing doses.
— Active phases of rheumatic systemic diseases: systemic lupus erythematosus 6-16 mg/day.
— Severely progressive form of active rheumatoid arthritis: in rapidly destructive forms 12-16 mg/day, in extra-articular manifestations 6-12 mg/day
— Severe infectious diseases, toxic states (e.g. tuberculosis, typhoid): 4-20 mg for a few days, only with concomitant anti-infective therapy.
— Palliative treatment of malignant tumours: initially 8-16 mg/day, in prolonged treatment 4-12 mg/day.
— Congenital adrenogenital syndrome in adulthood: 0.25-0.75 mg/day as a single dose. If necessary, addition of a mineralcorticoid (fludrocortisone). In cases of particular physical stress (e.g. trauma, surgery), intercurrent infections, etc., a 2- to 3-fold dose increase may be required and under extreme stress (e.g. birth) a 10-fold increase.
The tablets should not be split to adjust doses. If patients need a dose that cannot be provided by one or more tablets of 0.5mg, other appropriate formulations should be used.
Method of administration
The tablets should be taken during or after a meal. They should be swallowed whole, with a sufficient amount of liquid. The daily dose should be administered as a single dose in the morning, if possible (circadian therapy). In patients who require a high-dose therapy because of their disease, multiple daily dosing is often required to achieve maximum effect.
Depending on the underlying disease, clinical symptoms and response to therapy, the dose can be reduced at a faster or slower rate and the therapy stopped, or the patient is stabilised on a maintenance dose as low as possible and, if necessary, adrenal axis monitored. Basically, the dose and duration of treatment should be kept as high and long as necessary, but as low and short as possible. In principle, the dose should be reduced gradually.
In long-term therapy which is deemed necessary following initial treatment, patients should be switched to prednisone/prednisolone, because this leads to lower adrenal suppression.
In hypothyroidism or liver cirrhosis, low doses may be sufficient or a dose reduction may be necessary.
OZURDEX must be administered by a qualified ophthalmologist experienced in intravitreal injections.
Posology
The recommended dose is one OZURDEX implant to be administered intra-vitreally to the affected eye. Administration to both eyes concurrently is not recommended.
DME
Patients treated with OZURDEX who have experienced an initial response and in the physician’s opinion may benefit from retreatment without being exposed to significant risk should be considered for retreatment.
Retreatment may be performed after approximately 6 months if the patient experiences decreased vision and/or an increase in retinal thickness, secondary to recurrent or worsening diabetic macular oedema.
There is currently no experience of the efficacy or safety of repeat administrations in DME beyond 7 implants.
RVO and uveitis
Repeat doses should be considered when a patient experiences a response to treatment followed subsequently by a loss in visual acuity and in the physician’s opinion may benefit from retreatment without being exposed to significant risk.
Patients who experience and retain improved vision should not be retreated. Patients who experience deterioration in vision, which is not slowed by OZURDEX, should not be retreated.
There is only very limited information on repeat dosing intervals less than 6 months.
For information concerning the current safety experience of repeat administrations beyond 2 implants in posterior segment non-infectious uveitis and
Patients should be monitored following the injection to permit early treatment if an infection or increased intraocular pressure occurs.
Special populations
Elderly (>65 years old)
No dose adjustment is required for elderly patients.
Renal impairment
OZURDEX has not been studied in patients with renal impairment however no special considerations are needed in this population.
Hepatic impairment
OZURDEX has not been studied in patients with hepatic impairment; however no special considerations are needed in this population.
Paediatric population
There is no relevant use of OZURDEX in the paediatric population in
— diabetic macular oedema
— macular oedema following either Branch Retinal Vein Occlusion (BRVO) or Central Retinal Vein Occlusion (CRVO)
The safety and efficacy of OZURDEX in uveitis in the paediatric population have not been established. No data are available.
Method of administration
OZURDEX is a single-use intravitreal implant in applicator for intravitreal use only.
Each applicator can only be used for the treatment of a single eye.
The intravitreal injection procedure should be carried out under controlled aseptic conditions which include the use of sterile gloves, a sterile drape, and a sterile eyelid speculum (or equivalent).
The patient should be instructed to self-administer broad spectrum antimicrobial drops daily for 3 days before and after each injection. Before the injection, the periocular skin, eyelid and ocular surface should be disinfected (using for example drops of povidone iodine 5% solution on the conjunctiva as it was done in the clinical trials for the approval of OZURDEX) and adequate local anaesthesia should be administered. Remove the foil pouch from the carton and examine for damage. Then, in a sterile field, open the foil pouch and gently place the applicator on a sterile tray. Carefully remove the cap from the applicator. Once the foil pouch is opened the applicator should be used immediately.
Hold the applicator in one hand and pull the safety tab straight off the applicator. Do not twist or flex the tab. With the bevel of the needle up away from the sclera, advance the needle about 1 mm into the sclera then redirect toward the centre of the eye into the vitreous cavity until the silicone sleeve is against the conjunctiva. Slowly press the actuator button until an audible click is noted. Before withdrawing the applicator from the eye, make sure that the actuator button is fully pressed and has locked flush with the applicator surface. Remove the needle in the same direction as used to enter the vitreous.
Immediately after injecting OZURDEX, use indirect ophthalmoscopy in the quadrant of injection to confirm successful implantation. Visualisation is possible in the large majority of cases. In cases in which the implant cannot be visualised, take a sterile cotton bud and lightly depress over the injection site to bring the implant into view.
Following the intravitreal injection patients should continue to be treated with a broad spectrum antimicrobial.
Adults, adolescents, and children (2 years of age and above)
The frequency of instillation of drops and the duration of treatment will vary depending upon the severity of the underlying condition and the response to treatment.
Severe inflammations require one to two drops instilled into the eye every thirty to sixty minutes until a satisfactory response occurs.
Subconjunctival or systemic steroid therapy should be considered if there is no response. When a favourable response has been observed reduce the dosage towards one drop every four hours.
Nasolacrimal occlusion or gently closing the eyelid after administration is recommended. This may reduce the systemic absorption of medicinal products administered via the ocular route and result in a decrease in systemic adverse reactions.
Paediatric patients
The safety and efficacy of this product has not been established in children below 2 years of age.
Contraindications
The information provided in Contraindications of Onadron
is based on data of another medicine with exactly the same composition as the Onadron.
. Be careful and be sure to specify the information on the section Contraindications in the instructions to the drug Onadron directly from the package or from the pharmacist at the pharmacy.
more…
Eye drops; Eye suspension; Injection; Pills; Solution for intravenous and intramuscular injection; Substance-powder
Coated tablet; Intravitreal implant in applicator; Имплантат для интравитреального введения
Eye ointment
—
— Active or suspected ocular or periocular infection including most viral diseases of the cornea and conjunctiva, including active epithelial herpes simplex keratitis (dendritic keratitis), vaccinia, varicella, mycobacterial infections, and fungal diseases.
— Advanced glaucoma which cannot be adequately controlled by medicinal products alone.
— Aphakic eyes with ruptured posterior lens capsule.
— Eyes with Anterior Chamber Intraocular Lens (ACIOL), iris or transscleral fixated intraocular lens and ruptured posterior lens capsule.
— Vaccinia, varicella, or other viral diseases of cornea and conjunctiva (except herpes zoster keratitis)
— Herpes simplex keratitis
— Fungal diseases of ocular structures
— Mycobacterial ocular infections
— Acute, untreated bacterial infections
—
Special warnings and precautions for use
The information provided in Special warnings and precautions for use of Onadron
is based on data of another medicine with exactly the same composition as the Onadron.
. Be careful and be sure to specify the information on the section Special warnings and precautions for use in the instructions to the drug Onadron directly from the package or from the pharmacist at the pharmacy.
more…
Eye drops; Eye suspension; Injection; Pills; Solution for intravenous and intramuscular injection; Substance-powder
Coated tablet; Intravitreal implant in applicator; Имплантат для интравитреального введения
Eye ointment
Depending on the dose and duration of therapy, adrenocortical insufficiency caused by glucocorticoid therapy can continue for several months and in individual cases more than a year after cessation of therapy. In cases of particular physical stress situations (trauma, surgery, childbirth, etc.) during treatment with Onadron Krka, a temporary increase in dose may be required. Because of the potential risk in stress situations, patients on extended therapy should be issued a steroid card. Also in prolonged adrenal insufficiency after cessation of treatment, the administration of glucocorticoids may be necessary in physical stress situations. In case of intended withdrawal, treatment-induced acute adrenal insufficiency may be minimized by slow dose reduction.
Through immunosuppression, treatment with Onadron Krka can lead to an increased risk of bacterial, viral, parasitic, opportunistic and fungal infections. It can mask the symptoms of an existing or developing infection, thereby making a diagnosis more difficult. Latent infections, like tuberculosis or hepatitis B, can be reactivated.
Treatment with Onadron Krka should only be implemented in the event of the strongest indications and, if necessary, additional targeted anti-infective treatment administered for the following illnesses:
— Acute viral infections (Herpes zoster, Herpes simplex, Varicella, herpetic keratitis)
— HBsAG-positive chronic active hepatitis
— Approximately 8 weeks prior to 2 weeks after vaccinations with live vaccines
— Systemic mycoses and parasitoses (e.g. nematodes)
— In patients with suspected or confirmed strongyloidiasis (infection with threadworms), glucocorticoids can lead to activation and mass proliferation of these parasites
— Poliomyelitis
— Lymphadenitis after BCG vaccination
— Acute and chronic bacterial infections
— In a history of tuberculosis (reactivation risk), use only under tuberculostatic protection
In addition, treatment with Onadron Krka should only be implemented under strong indications and, if necessary, additional specific treatment must be implemented for:
— Gastrointestinal ulcers
— Osteoporosis
— Severe cardiac insufficiency
— High blood pressure that is difficult to regulate
— Diabetes mellitus that is difficult to regulate
— Psychiatric disorders (also in the past), including suicidality: neurological or psychiatric monitoring is recommended
— Narrow- and wide-angle glaucoma, ophthalmic monitoring and adjunctive therapy are recommended
— Corneal ulcerations and corneal injuries, ophthalmic monitoring and adjunctive therapy are recommended
Because of the risk of an intestinal perforation, Onadron Krka may only be used under urgent indication and under appropriate monitoring for:
— Severe ulcerative colitis with threatened perforation, possibly without peritoneal irritation
— Diverticulitis
— Enteroenterostomy (immediately postoperatively)
Signs of peritoneal irritation after gastrointestinal perforation may be absent in patients receiving high doses of glucocorticoids.
The possibility of a higher need for insulin or oral antidiabetics must be taken into consideration when administering Onadron Krka to diabetics.
Regular blood pressure monitoring is necessary during treatment with Onadron Krka, particularly during administration of higher doses and in patients with high blood pressure that is difficult to regulate.
Because of the risk of deterioration, patients with severe cardiac insufficiency should be carefully monitored.
With high doses of Onadron bradycardia may occur.
Severe anaphylactic reactions may occur.
The risk of tendon disorders, tendinitis and tendon rupture is increased when fluoroquinolones and glucocorticoids are administered together.
A concurrent myasthenia gravis may initially worsen during treatment with Onadron Krka.
Vaccinations with inactivated vaccines are generally possible. However, it should be noted that the immune response and thus the vaccine may be compromised at higher doses of corticosteroids.
During long-term therapy with Onadron Krka, regular medical checkups (including ophthalmologic every three months) are indicated.
At high doses, sufficient calcium intake and sodium restriction should be ensured and serum potassium levels should be monitored.
Depending on the dose and duration of treatment, a negative effect on calcium metabolism can be expected; therefore, the prevention of osteoporosis is recommended. This applies especially to patients with concomitant risk factors, such as familial predisposition, advanced age, postmenopausal period, insufficient protein and calcium intake, heavy smoking, excessive alcohol consumption and lack of physical activity. Prevention consists of sufficient calcium and vitamin D intake and physical activity. In already existing osteoporosis, additional drug therapy should be considered.
Upon termination of long-term administration of glucocorticoids, the following risks must be taken into account: exacerbation or relapse of the underlying disease, acute adrenal insufficiency, cortisone withdrawal syndrome.
Certain viral diseases (chickenpox, measles) may be very severe in patients treated with glucocorticoids. Immunocompromised patients without previous chickenpox or measles infection are particularly at risk. If these patients have contact with people infected with measles or chickenpox while undergoing treatment with Onadron Krka, a preventative treatment should be introduced, if necessary.
In post marketing experience tumour lysis syndrome (TLS) has been reported in patients with haematological malignancies following the use of Onadron alone or in combination with other chemotherapeutic agents. Patient at high risk of TLS, such as patients with high proliferative rate, high tumour burden, and high sensitivity to cytotoxic agents, should be monitored closely and appropriate precaution taken.
Visual disturbance
Visual disturbance may be reported with systemic and topical corticosteroid use. If a patient presents with symptoms such as blurred vision or other visual disturbances, the patient should be considered for referral to an ophthalmologist for evaluation of possible causes which may include cataract, glaucoma or rare diseases such as central serous chorioretinopathy (CSCR) which have been reported after use of systemic and topical corticosteroids.
Paediatric population
In the growth phase of children, the benefit-risk balance of treatment with Onadron Krka should be carefully weighed.
Therapy should be of limited duration or in case of long-term therapy, it should be carried out alternatingly.
Preterm neonates: Available evidence suggests long-term neurodevelopmental adverse events after early treatment (< 96 hours) of premature infants with chronic lung disease at starting doses of 0.25mg/kg twice daily.
Elderly patients
Because elderly patients are at an increased risk of osteoporosis, the benefit-risk balance of treatment with Onadron Krka should be carefully weighed.
Note
The use of Onadron Krka can lead to positive results in doping controls.
Onadron Krka contains lactose. Patients with rare hereditary problems of galactose intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption should not take this medicine.
Intravitreous injections, including those with OZURDEX, can be associated with endophthalmitis, intraocular inflammation, increased intraocular pressure and retinal detachment. Proper aseptic injection techniques must always be used. In addition, patients should be monitored following the injection to permit early treatment if an infection or increased intraocular pressure occurs. Monitoring may consist of a check for perfusion of the optic nerve head immediately after the injection, tonometry within 30 minutes following the injection, and biomicroscopy between two and seven days following the injection.
Patients must be instructed to report any symptoms suggestive of endophthalmitis or any of the above mentioned events without delay, e.g. eye pain, blurred vision etc..
All patients with posterior capsule tear, such as those with a posterior lens (e.g. due to cataract surgery), and/or those who have an iris opening to the vitreous cavity (e.g. due to iridectomy) with or without a history of vitrectomy, are at risk of implant migration into the anterior chamber. Implant migration to the anterior chamber may lead to corneal oedema. Persistent severe corneal oedema could progress to the need for corneal transplantation. Other than those patients contraindicated where OZURDEX should not be used, OZURDEX should be used with caution and only following a careful risk benefit assessment. These patients should be closely monitored to allow for early diagnosis and management of device migration.
Use of corticosteroids, including OZURDEX, may induce cataracts (including posterior subcapsular cataracts), increased IOP, steroid induced glaucoma and may result in secondary ocular infections.
In the 3 year DME clinical studies, 59% of patients with a phakic study eye treated with OZURDEX underwent cataract surgery in the study eye.
After the first injection the incidence of cataract appears higher in patients with non-infectious uveitis of the posterior segment compared with BRVO/CRVO patients. In BRVO/CRVO clinical studies, cataract was reported more frequently in patients with phakic lens receiving a second injection. Only 1 patient out of 368 required cataract surgery during the first treatment and 3 patients out of 302 during the second treatment. In the non-infectious uveitis study, 1 patient out of the 62 phakic patients underwent cataract surgery after a single injection.
The prevalence of conjunctival haemorrhage in patients with non-infectious uveitis of the posterior segment appears to be higher compared with BRVO/CRVO and DME. This could be attributable to the intravitreous injection procedure or to concomitant use of topical and/or systemic corticosteroid or Non-steroidal anti-inflammatory medications. No treatment is required since spontaneous resolution occurs.
As expected with ocular steroid treatment and intravitreal injections, increases in intraocular pressure (IOP) may be seen. The rise in IOP is normally manageable with IOP lowering medication. Of the patients experiencing an increase of IOP of >10 mmHg from baseline, the greatest proportion showed this IOP increase between 45 and 60 days following an injection. Therefore, regular monitoring of IOP, irrespective of baseline IOP, is required and any elevation should be managed appropriately post-injection as needed. Patients of less than 45 years of age with macular oedema following Retinal Vein Occlusion or inflammation of the posterior segment of the eye presenting as non-infectious uveitis are more likely to experience increases in IOP.
Corticosteroids should be used cautiously in patients with a history of ocular viral (e.g. herpes simplex) infection and not be used in active ocular herpes simplex.
The safety and efficacy of OZURDEX administered to both eyes concurrently have not been studied. Therefore administration to both eyes concurrently is not recommended.
OZURDEX has not been studied in patients with macular oedema secondary to RVO with significant retinal ischemia. Therefore OZURDEX is not recommended.
A limited number of subjects with Type 1 diabetes were investigated in the Phase 3 studies, and the response to OZURDEX in these subjects was not significantly different to those subjects with Type 2 diabetes.
In RVO, anti-coagulant therapy was used in 2% of patients receiving OZURDEX; there were no reports of haemorrhagic adverse events in these patients. In DME, anti-coagulant therapy was used in 8% of patients. Among patients who used anti-coagulant therapy, the frequency of haemorrhagic adverse events was similar in the OZURDEX and sham groups (29% vs 32%). Among patients who did not use anti-coagulant therapy, 27% of OZURDEX treated patients reported haemorrhagic adverse events compared to 20% in the sham group. Vitreous haemorrhage was reported in a higher proportion of patients treated with OZURDEX who received anti-coagulant therapy (11%) compared with those not receiving anticoagulant therapy (6%).
Anti-platelet medicinal products, such as clopidogrel, were used at some stage during the clinical studies in up to 56% of patients. For patients using concomitant and anti-platelet medication, haemorrhagic adverse events were reported in a slightly higher proportion of patients injected with OZURDEX (up to 29%) compared with the sham group (up to 23%), irrespective of indication or number of treatments. The most common haemorrhagic adverse event reported was conjunctival haemorrhage (up to 24%).
OZURDEX should be used with caution in patients taking anti-coagulant or anti-platelet medicinal products.
— For ocular use only.
— Prolonged use of topical ophthalmic corticosteroids may result in ocular hypertension and/or glaucoma, with damage to the optic nerve, reduced visual acuity, visual field defects and posterior subcapsular cataract formation. In patients receiving prolonged ophthalmic corticosteroid therapy, intraocular pressure and the lens should be checked routinely and frequently, particularly in patients with a history or presence of glaucoma. This is especially important in paediatric patients as the risk of corticosteroid-induced ocular hypertension may be greater in children and may occur earlier than in adults. The risk of corticosteroid-induced raised intraocular pressure and/or cataract formation is increased in predisposed patients (e.g. diabetes).
— Topical corticosteroids should not be used for longer than one week except under ophthalmic supervision, with regular checks of intraocular pressure.
— Cushing’s syndrome and/or adrenal suppression associated with systemic absorption of ocular dexamethasone may occur after intensive or long-term continuous therapy in predisposed patients, including children and patients treated with CYP3A4 inhibitors (including ritonavir and cobicistat). In these cases, treatment should be progressively discontinued.
— Corticosteroids may reduce resistance to and aid in the establishment of bacterial, viral and fungal infections and mask the clinical signs of infections. In such cases antibiotic therapy is mandatory. Fungal infection should be suspected in patients with persistent corneal ulceration and corticosteroids therapy should be discontinued if fungal infection occurs.
— Topical ophthalmic corticosteroids may slow corneal wound healing. Topical NSAIDs are also known to slow or delay healing. Concomitant use of topical NSAIDs and topical steroids may increase the potential for healing problems..
— In those diseases causing thinning of the cornea or sclera, perforations have been known to occur with the use of topical corticosteroids.
— Visual disturbance may be reported with systemic and topical corticosteroid use. If a patient presents with symptoms such as blurred vision or other visual disturbances, the patient should be considered for referral to an ophthalmologist for evaluation of possible causes which may be cataract, glaucoma or rare diseases such as central serous chorioretinopathy (CSCR) which have been reported after use of systemic and topical corticosteroids.
— Contact lens wear is not recommended during treatment of an ocular inflammation.
— Additionally, this product contains benzalkonium chloride which may cause eye irritation and is known to discolour soft contact lenses. Avoid contact with soft contact lenses. Patients must be instructed to remove contact lenses prior to application of Onadron and wait at least 15 minutes before reinsertion.
— There is no evidence of safety in use in children under two years of age.
Effects on ability to drive and use machines
The information provided in Effects on ability to drive and use machines of Onadron
is based on data of another medicine with exactly the same composition as the Onadron.
. Be careful and be sure to specify the information on the section Effects on ability to drive and use machines in the instructions to the drug Onadron directly from the package or from the pharmacist at the pharmacy.
more…
Eye drops; Eye suspension; Injection; Pills; Solution for intravenous and intramuscular injection; Substance-powder
Coated tablet; Intravitreal implant in applicator; Имплантат для интравитреального введения
Eye ointment
There have been no studies on the effects on the ability to drive and use machines.
OZURDEX may have a moderate influence on the ability to drive and use machines. Patients may experience temporarily reduced vision after receiving OZURDEX by intravitreal injection. They should not drive or use machines until this has resolved.
Onadron has no or negligible influence on the ability to drive and use machines. As with any topical ophthalmic medicinal product, temporary blurred vision or other visual disturbances may affect the ability to drive or use machines. If blurred vision occurs upon instillation, the patient must wait until the vision clears before driving or using machinery.
Undesirable effects
The information provided in Undesirable effects of Onadron
is based on data of another medicine with exactly the same composition as the Onadron.
. Be careful and be sure to specify the information on the section Undesirable effects in the instructions to the drug Onadron directly from the package or from the pharmacist at the pharmacy.
more…
Eye drops; Eye suspension; Injection; Pills; Solution for intravenous and intramuscular injection; Substance-powder
Coated tablet; Intravitreal implant in applicator; Имплантат для интравитреального введения
Eye ointment
— Very common (> 1/10)
— Common (> 1/100 to < 1/10)
— Uncommon (> 1/1,000 to < 1/100)
— Rare (> 1/10,000 to < 1/1,000)
— Very rare (< 1/10,000)
— Not known (cannot be estimated from the available data)
Hormone replacement therapy:
Low risk of undesirable effects with the use of recommended doses.
Pharmacotherapy:
The following undesirable effects may occur; they are highly dependent on the dose and duration of treatment, so their frequency cannot be specified:
|
Infections and infestations |
Masking of infections, manifestation and exacerbation of viral infections, fungal infections, bacterial, parasitic and opportunistic infections, activation of strongyloidiasis. |
|
Blood and lymphatic system disorders |
Moderate leukocytosis, lymphocytopenia, eosinopenia, polycythemia. |
|
Immune system disorders |
Hypersensitivity reactions (e.g. drug eruption), severe anaphylactic reactions, such as arrhythmias, bronchospasm, hypo- or hypertension, circulatory collapse, cardiac arrest, weakening of the immune system. |
|
Endocrine disorders |
Adrenal suppression and induction of Cushing’s syndrome (typical symptoms: moon face, central obesity and plethora). |
|
Metabolism and nutrition disorders |
Sodium retention with oedema, increased potassium excretion (risk of arrhythmias), weight gain, reduced glucose tolerance, diabetes mellitus, hypercholesterolemia and hypertriglyceridemia, increased appetite. |
|
Psychiatric disorders |
Depression, irritability, euphoria, increased drive, psychoses, mania, hallucinations, emotional lability, anxiety, sleep disorders, suicidality. |
|
Nervous system disorders |
Pseudotumor cerebri, manifestation of latent epilepsy, increase in seizure susceptibility in manifest epilepsy. |
|
Eye disorders |
|
|
Vascular disorders |
Hypertension, increased risk of atherosclerosis and thrombosis, vasculitis (also as withdrawal syndrome after long-term therapy), increased capillary fragility. |
|
Gastrointestinal disorders |
Gastrointestinal ulcers, gastrointestinal bleeding, pancreatitis, stomach discomfort. |
|
Skin and subcutaneous tissue disorders |
Striae rubra, atrophy, telangiectasias, petechiae, ecchymosis, hypertrichosis, steroid acne, rosacea-like (perioral) dermatitis, changes in skin pigmentation. |
|
Musculoskeletal and connective tissue disorders |
Myopathy, muscle atrophy and weakness, osteoporosis (dose-dependent, possible also in short-term administration), aseptic bone necrosis, tendon disorders, tendinitis, tendon rupture, epidural lipomatosis, growth inhibition in children. Note: Too rapid dose reduction after long-term treatment may cause symptoms such as muscle and joint pain. |
|
Reproductive system and breast disorders |
Disorders of sexual hormone secretion (consequently: irregular menstruation up to amenorrhea, hirsutism, impotence). |
|
General disorders and administration site conditions |
Delayed wound healing. |
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard.
Summary of the safety profile
The most commonly-reported adverse events reported following treatment with OZURDEX are those frequently observed with ophthalmic steroid treatment or intravitreal injections (elevated IOP, cataract formation and conjunctival or vitreal haemorrhage respectively).
Less frequently reported, but more serious, adverse reactions include endophthalmitis, necrotizing retinitis, retinal detachment and retinal tear.
With the exception of headache and migraine, no systemic adverse drug reactions were identified with the use of OZURDEX.
Tabulated list of adverse reactions
The adverse reactions considered related to OZURDEX treatment from the Phase III clinical trials (DME, BRVO/CRVO and uveitis) and spontaneous reporting are listed by MedDRA System organ class in the table below using the following convention:
Very common (> 1/10); common (>1/100 to <1/10); uncommon (>1/1,000 to <1/100); rare (>1/10,000 to <1/1,000); very rare (<1/10,000). Within each frequency grouping, adverse reactions are presented in order of decreasing seriousness.
Table 1 Adverse reactions
|
System organ class |
Frequency |
Adverse reaction |
|
Nervous system disorders |
Common |
Headache |
|
Uncommon |
Migraine |
|
|
Eye disorders |
Very common |
Intraocular pressure increased**, cataract**, conjunctival haemorrhage* |
|
Common |
Ocular hypertension, cataract subcapsular, vitreous haemorrhage**, visual acuity reduced*, visual impairment/ disturbance, vitreous detachment*, vitreous floaters*, vitreous opacities*, blepharitis, eye pain*, photopsia*, conjunctival oedema* conjunctival hyperaemia* |
|
|
Uncommon |
Necrotizing retinitis, endophthalmitis*, glaucoma, retinal detachment*, retinal tear*, hypotony of the eye*, anterior chamber inflammation*, anterior chamber cells/ flares*, abnormal sensation in eye*, eyelids pruritus, scleral hyperaemia* |
|
|
General disorders and administration site conditions |
Uncommon |
Device dislocation* (migration of implant) with or without corneal oedema (see also section 4.4), complication of device insertion* (implant misplacement) |
* indicates adverse reactions considered to be related to the intravitreal injection procedure (the frequency of these adverse reactions is proportional to the number of treatments given).
** in a 24-month real world observational study in the treatment of macular oedema following RVO and non-infectious uveitis affecting the posterior segment of the eye these adverse events were reported more frequently among patients who received >2 injections vs patients who received ≤2 injections; cataract formation (24.7% vs 17.7%), cataract progression (32.0% vs 13.1%), vitreous haemorrhage (6.0% vs 2.0%), and increased IOP (24.0% vs 16.6%).
Description of selected adverse reactions
Diabetic Macular Oedema
The clinical safety of OZURDEX in patients with diabetic macular oedema was assessed in two phase 3 randomized, double-masked, sham-controlled studies. In both studies, a total of 347 patients were randomized and received OZURDEX and 350 patients received sham.
The most frequently reported adverse reactions across the entire study period in the study eye of patients who received OZURDEX were cataract and elevated IOP (see below).
In the 3 year DME clinical studies, at baseline, 87% of patients with a phakic study eye treated with OZURDEX had some degree of lens opacification/ early cataract. The incidence of all observed cataract types (i.e. cataract cortical, cataract diabetic, cataract nuclear, cataract subcapsular, cataract lenticular, cataract) was 68% in OZURDEX treated patients with a phakic study eye across the 3 year studies. 59% of patients with a phakic study eye required cataract surgery by the 3 year final visit, with the majority performed in the 2nd and 3rd years.
Mean IOP in the study eye at baseline was the same in both treatment groups (15.3 mmHg). The mean increase from baseline IOP did not exceed 3.2 mmHg across all visits in the OZURDEX group with the mean IOP peaking at the 1.5 month visit post injection, and returning to approximately baseline levels by month 6 following each injection. The rate and magnitude of IOP elevation following OZURDEX treatment did not increase upon repeated injection of OZURDEX.
28% of patients treated with OZURDEX had a > 10 mm Hg IOP increase from baseline at one or more visits during the study. At baseline 3% of patients required IOP-lowering medication(s). Overall, 42% of patients required IOP-lowering medications in the study eye at some stage during the 3 year studies, with the majority of these patients requiring more than one medication. Peak usage (33%) occurred during the first 12 months and remained similar from year to year.
A total of 4 patients (1%) treated with OZURDEX had procedures in the study eye for the treatment of IOP elevation. One patient treated with OZURDEX required incisional surgery (trabeculectomy) to manage the steroid-induced IOP elevation, 1 patient had a trabeculectomy owing to anterior chamber fibrin blocking the aqueous outflow leading to increased IOP, 1 patient had an iridotomy for narrow angle glaucoma and 1 patient had iridectomy due to cataract surgery. No patient required removal of the implant by vitrectomy to control IOP.
BRVO/CRVO
The clinical safety of OZURDEX in patients with macular oedema following central or branch retinal vein occlusion has been assessed in two Phase III randomised, double-masked, sham-controlled studies. A total of 427 patients were randomised to receive OZURDEX and 426 to receive sham in the two Phase III studies. A total of 401 patients (94 %) randomised and treated with OZURDEX completed the initial treatment period (up to day 180).
A total of 47.3 % of patients experienced at least one adverse reaction. The most frequently reported adverse reactions in patients who received OZURDEX were increased intraocular pressure (24.0 %) and conjunctival haemorrhage (14.7 %).
The adverse reaction profile for BRVO patients was similar to that observed for CRVO patients although the overall incidence of adverse reactions was higher for the subgroup of patients with CRVO.
Increased intraocular pressure (IOP) with OZURDEX peaked at day 60 and returned to baseline levels by day 180. Elevations of IOP either did not require treatment or were managed with the temporary use of topical IOP-lowering medicinal products. During the initial treatment period, 0.7 % (3/421) of the patients who received OZURDEX required laser or surgical procedures for management of elevated IOP in the study eye compared with 0.2 % (1/423) with sham.
The adverse reaction profile of 341 patients analysed following a second injection of OZURDEX, was similar to that following the first injection. A total of 54 % of patients experienced at least one adverse reaction. The incidence of increased IOP (24.9 %) was similar to that seen following the first injection and likewise returned to baseline by open-label day 180. The overall incidence of cataracts was higher after 1 year compared to the initial 6 months.
Uveitis
The clinical safety of OZURDEX in patients with inflammation of the posterior segment of the eye presenting as non-infectious uveitis, has been assessed in a single, multicentre, masked, randomised study.
A total of 77 patients were randomised to receive OZURDEX and 76 to receive Sham. A total of 73 patients (95%) randomised and treated with OZURDEX completed the 26-week study.
The most frequently reported adverse reactions in the study eye of patients who received OZURDEX were conjunctival haemorrhage (30.3%), increased intraocular pressure (25.0%) and cataract (11.8%).
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme
Website: www.mhra.gov.uk/yellowcard.
Summary of the safety profile
In clinical trials, the most common adverse reaction was ocular discomfort.
Tabulated list of adverse reactions
The following adverse reactions are classified according to the following convention: very common (> 1/10), common (> 1/100 to <1/10), uncommon (>1/1,000 to <1/100), rare (>1/10,000 to <1/1,000), very rare (<1/10,000), or not known (cannot be estimated from the available data). Within each frequency-grouping, adverse reactions are presented in order of decreasing seriousness. The adverse reactions have been observed during clinical trials and post-marketing experience with Onadron.
|
System Organ Classification |
MedDRA Preferred Term (v. 12.0) |
|
Immune system disorders |
Not known: hypersensitivity |
|
Endocrine disorders |
Not known: Cushing’s syndrome, adrenal suppression |
|
Nervous system disorders |
Uncommon: dysgeusia Not known: dizziness, headache |
|
Eye disorders |
Common: ocular discomfort Uncommon: keratitis, conjunctivitis, keratoconjunctivitis sicca, corneal staining, photophobia, vision, blurred (see also section 4.4), eye pruritus, foreign body sensation in eyes, lacrimation increased, abnormal sensation in eyes, eyelid margin crusting, eye irritation, ocular hyperaemia Not known: intraocular pressure increased, visual acuity reduced, corneal erosion, eyelid ptosis, eye pain, mydriasis |
Description of selected adverse reactions
Prolonged topical ophthalmic corticosteroids may result in increased intraocular pressure with damage to the optic nerve, reduced visual acuity and visual field defects, and to posterior subcapsular cataract formation.
Due to the corticosteroid component, in diseases causing thinning of the cornea or sclera there is a higher risk for perforation especially after long treatments.
Corticosteroids may reduce resistance to and aid in the establishment of infections.
Cases of corneal calcification have been reported very rarely in association with the use of phosphate containing eye drops in some patients with significantly damaged corneas.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the national reporting system:
United Kingdom
Yellow Card Scheme
Website: www.mhra.gov.uk/yellowcard
Overdose
The information provided in Overdose of Onadron
is based on data of another medicine with exactly the same composition as the Onadron.
. Be careful and be sure to specify the information on the section Overdose in the instructions to the drug Onadron directly from the package or from the pharmacist at the pharmacy.
more…
Eye drops; Eye suspension; Injection; Pills; Solution for intravenous and intramuscular injection; Substance-powder
Coated tablet; Intravitreal implant in applicator; Имплантат для интравитреального введения
Eye ointment
Symptoms
Acute intoxications with Onadron are not known. In case of chronic overdosing, an increase in undesirable effects, especially endocrine, metabolic and electrolyte-related effects, can be expected.
Management
There is no known antidote to Onadron.
If an overdose occurs, intraocular pressure should be monitored and treated, if deemed necessary by the attending physician.
Long-term intensive topical use may lead to systemic effects. Oral ingestion of the contents of the bottle (up to 10 mls) is unlikely to lead to any serious adverse effects.
An ocular overdose of Onadron can be flushed from the eye(s) with lukewarm water.
Pharmacodynamic properties
The information provided in Pharmacodynamic properties of Onadron
is based on data of another medicine with exactly the same composition as the Onadron.
. Be careful and be sure to specify the information on the section Pharmacodynamic properties in the instructions to the drug Onadron directly from the package or from the pharmacist at the pharmacy.
more…
Eye drops; Eye suspension; Injection; Pills; Solution for intravenous and intramuscular injection; Substance-powder
Coated tablet; Intravitreal implant in applicator; Имплантат для интравитреального введения
Eye ointment
Pharmacotherapeutic group: corticosteroids for systemic use, glucocorticoids, ATC code: H02AB02.
Mechanism of action
Onadron is a mono-fluorinated glucocorticoid with pronounced anti-allergic, anti-inflammatory and membrane-stabilizing properties and effects on carbohydrate, protein and fat metabolism.
Onadron has an approximately 7.5 times greater glucocorticoid effect than prednisolone, and compared to hydrocortisone it is 30 times more effective, lacking mineralocorticoid effects.
Glucocorticoids, such as Onadron, exert their biological effects by activating the transcription of corticosteroid-sensitive genes. The anti-inflammatory, immunosuppressive and anti-proliferative effects are caused by decreased formation, release and activity of inflammatory mediators, by the inhibition of specific functions and the migration of inflammatory cells. In addition, the effect of sensitized T lymphocytes and macrophages on target cells may be prevented by corticosteroids.
When long-term corticoid treatment is required, the possibility of induction of transient adrenal insufficiency must be considered. The suppression of the hypothalamic-pituitary-adrenal axis also depends on individual factors.
Pharmacotherapeutic group: Ophthalmologicals, antiinflammatory agents, ATC code: S01BA01
Dexamethasone, a potent corticosteroid, has been shown to suppress inflammation by inhibiting oedema, fibrin deposition, capillary leakage, and phagocytic migration of the inflammatory response. Vascular Endothelial Growth Factor (VEGF) is a cytokine which is expressed at increased concentrations in the setting of macular oedema. It is a potent promoter of vascular permeability. Corticosteroids have been shown to inhibit the expression of VEGF. Additionally, corticosteroids prevent the release of prostaglandins, some of which have been identified as mediators of cystoid macular oedema.
Clinical efficacy and safety
Diabetic Macular Oedema
The efficacy of OZURDEX was assessed in two 3 year, multicentre, double-masked, randomised, sham-controlled, parallel studies of identical design which together comprised 1,048 patients (studies 206207-010 and 206207-011). A total of 351 were randomised to OZURDEX, 347 to dexamethasone 350 µg and 350 patients to sham.
Patients were eligible for retreatment based upon central subfield retinal thickness >175 microns by optical coherence tomography (OCT) or upon investigators interpretation of the OCT for any evidence of residual retinal edema consisting of intraretinal cysts or any regions of increased retinal thickening within or outside of the central subfield. Patients received up to 7 treatments at intervals no more frequently than approximately every 6 months.
Escape therapy was permitted at the investigators discretion at any stage but led to subsequent withdrawal from the studies.
A total of 36% of OZURDEX treated patients discontinued study participation for any reason during the study compared with 57% of sham patients. Discontinuation rates due to adverse events were similar across treatment and sham groups (13% vs 11%). Discontinuation due to lack of efficacy was lower in the OZURDEX group compared to sham (7% vs 24%).
The primary and key secondary endpoints for studies 206207-010 and 011 are presented in Table 2. The vision improvement in the DEX700 group was confounded by cataract formation. Vision improvement was re-established upon removal of cataract.
Table 2. Efficacy in studies 206207-010 and 206207-011 (ITT population)
|
Endpoint |
Study 206207-010 |
Study 206207-011 |
Pooled Studies 206207-010 and 206207-011 |
|||
|
DEX 700 N = 163 |
Sham N = 165 |
DEX 700 N = 188 |
Sham N = 185 |
DEX 700 N = 351 |
Sham N = 350 |
|
|
Mean BCVA average change over 3 years, AUC approach (letters) |
4.1 |
1.9 |
2.9 |
2.0 |
3.5 |
2.0 |
|
P-value |
0.016 |
0.366 |
0.023 |
|||
|
BCVA > 15-letter improvement from baseline at Year 3/Final (%) |
22.1 |
13.3 |
22.3 |
10.8 |
22.2 |
12.0 |
|
P-value |
0.038 |
0.003 |
< 0.001 |
|||
|
Mean BCVA change from baseline at year 3/final visit (letters) |
4.1 |
0.8 |
1.3 |
-0.0 |
2.6 |
0.4 |
|
P-value |
0.020 |
0.505 |
0.054 |
|||
|
OCT retinal thickness at center subfield mean average change over 3 years, AUC approach (µm) |
-101.1 |
-37.8 |
-120.7 |
-45.8 |
-111.6 |
-41.9 |
|
P-value |
<0.001 |
< 0.001 |
< 0.001 |
The primary and key secondary endpoints for the pooled analysis for pseudophakic patients are presented in Table 3.
Table 3. Efficacy in pseudophakic patients (pooled studies 206207-010 and 206207-011)
|
Endpoint |
DEX 700 N = 86 |
Sham N = 101 |
P-value |
|
Mean BCVA average change over 3 years, AUC approach (letters) |
6.5 |
1.7 |
< 0.001 |
|
BCVA > 15-letter improvement from baseline at Year 3/Final visit (%) |
23.3 |
10.9 |
0.024 |
|
Mean BCVA change from baseline at year 3/Final visit |
6.1 |
1.1 |
0.004 |
|
OCT retinal thickness at center subfield mean average change over 3 years, AUC approach (µm) |
-131.8 |
-50.8 |
< 0.001 |
The primary and key secondary endpoints for the pooled analysis for patients with any prior treatment are presented in Table 4.
Table 4. Efficacy in patients with any prior treatment (pooled studies 206207-010 and 206207-011)
|
Endpoint |
DEX 700 N = 247 |
Sham N = 261 |
P-value |
|
Mean BCVA average change over 3 years, AUC approach (letters) |
3.2 |
1.5 |
0.024 |
|
BCVA > 15-letter improvement from baseline at Year 3/Final visit (%) |
21.5 |
11.1 |
0.002 |
|
Mean BCVA change from baseline at year 3/Final visit |
2.7 |
0.1 |
0.055 |
|
OCT retinal thickness at center subfield mean average change over 3 years, AUC approach (µm) |
-126.1 |
-39.0 |
< 0.001 |
BRVO/CRVO
The efficacy of OZURDEX was assessed in two multicentre, double-masked, randomised, sham-controlled, parallel studies of identical design which together comprised 1,267 patients who were randomized to receive treatment with dexamethasone 350 µg or 700 µg implants or sham (studies 206207-008 and 206207-009). A total of 427 were randomised to OZURDEX, 414 to dexamethasone 350 µg and 426 patients to sham.
Based on the pooled analysis results, treatment with OZURDEX implants showed statistically significantly greater incidence of responders, defined as patients achieving a > 15 letter improvement from baseline in Best Corrected Visual Acuity (BCVA) at 90 days following injection of a single implant, when compared with sham (p < 0.001).
The proportion of patients achieving the primary efficacy measure of > 15 letter improvement from baseline in BCVA following injection of a single implant is shown in Table 5. A treatment effect was seen at the first observation time point of day 30. The maximum treatment effect was observed at day 60 and the difference in the incidence of responders was statistically significant favouring OZURDEX compared with sham at all time points to day 90 following injection. There continued to be a numerically greater proportion of responders for a > 15 letter improvement from baseline in BCVA in patients treated with OZURDEX compared with sham at day 180.
Table 5. Proportion of patients with > 15 letters improvement from baseline best corrected visual acuity in the study eye (pooled, ITT population)
|
Visit |
OZURDEX N = 427 |
Sham N = 426 |
|
Day 30 |
21.3 % a |
7.5% |
|
Day 60 |
29.3% a |
11.3% |
|
Day 90 |
21.8% a |
13.1% |
|
Day 180 |
21.5% |
17.6% |
a Proportion significantly higher with OZURDEX compared to sham (p < 0.001)
The mean change from baseline BCVA was significantly greater with OZURDEX compared to sham at all time points.
In each Phase III study and the pooled analysis, the time to achieve > 15 letters (3-line) improvement in BCVA cumulative response curves were significantly different with OZURDEX compared to sham (p < 0.001) with OZURDEX treated patients achieving a 3-line improvement in BCVA earlier than sham treated patients.
OZURDEX was numerically superior to sham in preventing vision loss as shown by a lower of proportion of patients experiencing deterioration of vision of > 15 letters in the OZURDEX group throughout the 6-month assessment period.
In each of the phase III studies and the pooled analysis, mean retinal thickness was significantly less, and the mean reduction from baseline was significantly greater, with OZURDEX (-207.9 microns) compared to sham (-95.0 microns) at day 90 (p < 0.001, pooled data). The treatment effect as assessed by BCVA at day 90 was thus supported by this anatomical finding. By Day 180 the mean retinal thickness reduction (-119.3 microns) compared with sham was not significant.
Patients who had a BCVA score of <84 OR retinal thickness > 250 microns by optical coherence tomography OCT and in the investigator’s opinion treatment would not put the patient at risk; were eligible to receive an OZURDEX treatment in an open label extension. Of the patients who were treated in the open label phase, 98% received an OZURDEX injection between 5 and 7 months after the initial treatment.
As for the initial treatment, peak response was seen at Day 60 in the open label phase. The cumulative response rates were higher throughout the open label phase in those patients receiving two consecutive OZURDEX injections compared with those patients who had not received an OZURDEX injection in the initial phase.
The proportion of responders at each time point was always greater after the second treatment compared with the first treatment. Whereas, delaying treatment for 6 months results in a lower proportion of responders at all time points in the open label phase when compared with those receiving a second OZURDEX injection.
Uveitis
The clinical efficacy of OZURDEX has been assessed in a single, multicentre, masked, randomised study for the treatment of non-infectious ocular inflammation of the posterior segment in patients with uveitis.
A total of 229 patients were randomised to receive dexamethasone 350 µg or 700 µg implants or sham. Of these, a total of 77 were randomised to receive OZURDEX, 76 to dexamethasone 350 µg and 76 to sham. A total of 95% of patients completed the 26-week study.
The proportion of patients with vitreous haze score of 0 in the study eye at week 8 (primary endpoint) was 4-fold higher with OZURDEX (46.8%) compared to Sham (11.8%), p < 0.001. Statistical superiority was maintained up to and including week 26 (p ≤ 0.014) as shown in Table 6.
The cumulative response rate curves (time to vitreous haze score of 0) were significantly different for the OZURDEX group compared to the Sham group (p < 0.001), with patients receiving dexamethasone showing an earlier onset and greater treatment response.
The reduction in vitreous haze was accompanied by an improvement in visual acuity. The proportion of patients with at least 15 letters improvement from baseline BCVA in the study eye at week 8 was more than 6-fold higher with OZURDEX (42.9%) compared to Sham (6.6%), p < 0.001. Statistical superiority was achieved at week 3 and maintained up to and including week 26 (p < 0.001) as shown in Table 6.
The percent of patients requiring escape medications from baseline to week 8 was nearly 3-fold less with OZURDEX (7.8%) compared to Sham (22.4%), p = 0.012.
Table 6. Proportion of patients with vitreous haze score of zero and > 15 letters improvement from baseline best corrected visual acuity in the study eye (ITT population)
|
Visit |
Vitreous Haze Score of Zero |
BCVA improvement from baseline of >15 letters |
||
|
DEX 700 N = 77 |
Sham N = 76 |
DEX 700 N = 77 |
Sham N = 76 |
|
|
Week 3 |
23.4% |
11.8% |
32.5%a |
3.9% |
|
Week 6 |
42.9%a |
9.2% |
41.6%a |
7.9% |
|
Week 8 |
46.8%a |
11.8% |
42.9%a |
6.6% |
|
Week 12 |
45.5%a |
13.2% |
41.6%a |
13.2% |
|
Week 16 |
40.3%b |
21.1% |
39.0%a |
13.2% |
|
Week 20 |
39.0%c |
19.7% |
40.3%a |
13.2% |
|
Week 26 |
31.2%d |
14.5% |
37.7%a |
13.2% |
a p < 0.001; b p = 0.010; c p = 0.009; d p = 0.014
Paediatric population
).
Pharmacotherapeutic Group: Ophthalmologicals: Anti-inflammatory Agents.
ATC Code S01B A01.
Dexamethasone has been demonstrated by animal and human studies based on oral application to possess approximately six to seven times the potency of prednisolone and at least 30 times the potency of cortisone. The potency of the compound is accomplished by the addition of a methyl radical and a fluorine atom to the prednisolone radical.
Pharmacokinetic properties
The information provided in Pharmacokinetic properties of Onadron
is based on data of another medicine with exactly the same composition as the Onadron.
. Be careful and be sure to specify the information on the section Pharmacokinetic properties in the instructions to the drug Onadron directly from the package or from the pharmacist at the pharmacy.
more…
Eye drops; Eye suspension; Injection; Pills; Solution for intravenous and intramuscular injection; Substance-powder
Coated tablet; Intravitreal implant in applicator; Имплантат для интравитреального введения
Eye ointment
Absorption and distribution
After oral administration, Onadron is rapidly and almost completely absorbed in the stomach and small intestine. Its bioavailability is 80-90%. Maximum blood levels are reached between 60 and 120 minutes. The binding of Onadron to plasma albumins is dose-dependent. At very high doses, the largest portion circulates freely in the blood. In hypoalbuminaemia the proportion of the unbound (active) corticoid rises.
Biotransformation
The average (serum) elimination half-life of Onadron in adults is 250 minutes (+ 80 minutes). Due to its long biological half-life of more than 36 hours, daily continuous administration of Onadron can lead to accumulation and overdosing.
Elimination
The elimination is largely renal in the form of free Onadron alcohol. Onadron is partly metabolised, the metabolites are excreted as glucuronates or sulfates, also mainly by the kidneys.
Renal and hepatic impairment
Renal function impairment has no relevant effect on the clearance of Onadron. However, the elimination half-life is prolonged in severe liver disease.
Plasma concentrations were obtained from a subset of 21 patients in the two RVO, 6-month efficacy studies prior to dosing and on days 7, 30, 60 and 90 following intravitreal injection of a single intravitreal implant containing 350 µg or 700 µg dexamethasone. Ninety-five percent of the plasma dexamethasone concentration values for the 350 µg dose group and 86% for the 700 µg dose group were below the lower limit of quantitation (0.05 ng/mL). The highest plasma concentration value of 0.094 ng/mL was observed in one subject from the 700 µg group. Plasma dexamethasone concentration did not appear to be related to age, body weight, or sex of patients.
Plasma concentrations were obtained from a subgroup of patients in the two DME pivotal studies prior to dosing and on days 1, 7, and 21, and months 1.5 and 3 following intravitreal injection of a single intravitreal implant containing 350 µg or 700 µg dexamethasone. One hundred percent of the plasma dexamethasone concentration values for the 350 µg dose group and 90% for the 700 µg dose group were below the lower limit of quantitation (0.05 ng/mL). The highest plasma concentration value of 0.102 ng/mL was observed in 1 subject from the 700 µg group. Plasma dexamethasone concentration did not appear to be related to age, body weight, or sex of patients.
In a 6-month monkey study following a single intravitreal injection of OZURDEX the dexamethasone vitreous humour Cmax was 100 ng/mL at day 42 post-injection and 5.57 ng/mL at day 91. Dexamethasone remained detectable in the vitreous at 6 months post-injection. The rank order of dexamethasone concentration was retina > iris > ciliary body > vitreous humour > aqueous humour > plasma.
In an in vitro metabolism study, following the incubation of [14C]-dexamethasone with human cornea, iris-ciliary body, choroid, retina, vitreous humour, and sclera tissues for 18 hours, no metabolites were observed. This is consistent with results from rabbit and monkey ocular metabolism studies.
Dexamethasone is ultimately metabolised to lipid and water soluble metabolites that can be excreted in bile and urine.
The OZURDEX matrix slowly degrades to lactic acid and glycolic acid through simple hydrolysis, then further degrades into carbon dioxide and water.
Dexamethasone is absorbed rapidly after oral administration with a half-life of about 190 minutes. Sufficient absorption may occur after topical application to the skin and eye to produce systemic effects. In plasma dexamethasone protein binding is less than for most other corticosteroids. Corticosteroids diffuse into tissue fluids and cerebrospinal fluid but transplacental diffusion in significant amounts has not been demonstrated. Corticosteroids are metabilised in the liver the kidney and excrete in the urine. Metabolism is similar to other corticosteroids. Intraocular penetration occurs in significant amounts and contributes to the effectiveness of dexamethasone in anterior segment inflammatory disease.
Pharmacotherapeutic group
The information provided in Pharmacotherapeutic group of Onadron
is based on data of another medicine with exactly the same composition as the Onadron.
. Be careful and be sure to specify the information on the section Pharmacotherapeutic group in the instructions to the drug Onadron directly from the package or from the pharmacist at the pharmacy.
more…
Eye drops; Eye suspension; Injection; Pills; Solution for intravenous and intramuscular injection; Substance-powder
Coated tablet; Intravitreal implant in applicator; Имплантат для интравитреального введения
Eye ointment
corticosteroids for systemic use, glucocorticoids, ATC code: H02AB02.
Ophthalmologicals, antiinflammatory agents, ATC code: S01BA01
Ophthalmologicals: Anti-inflammatory Agents.
Preclinical safety data
The information provided in Preclinical safety data of Onadron
is based on data of another medicine with exactly the same composition as the Onadron.
. Be careful and be sure to specify the information on the section Preclinical safety data in the instructions to the drug Onadron directly from the package or from the pharmacist at the pharmacy.
more…
Eye drops; Eye suspension; Injection; Pills; Solution for intravenous and intramuscular injection; Substance-powder
Coated tablet; Intravitreal implant in applicator; Имплантат для интравитреального введения
Eye ointment
Acute toxicity:
In mice and rats, the LD50 for Onadron after a single oral dose is 16 g/kg body and over 3 g/kg body weight, respectively, within the first 7 days. Following a single subcutaneous dose, the LD50 in mice is more than 700 mg/kg body weight and in rats about 120 mg/kg body weight, within the first 7 days.
Over a period of 21 days, these values become lower, which is interpreted as a consequence of serious infectious diseases caused by the hormone-induced immunosuppression.
Chronic toxicity:
There are no data on chronic toxicity in humans and animals. Corticoid-induced intoxications are not known. In longer-term treatment with doses above 1.5 mg/day, pronounced undesirable effects can be expected.
Mutagenic and tumorigenic potential:
The available study findings for glucocorticoids show no evidence of clinically relevant genotoxic properties.
Reproductive toxicity:
In animal studies, cleft palate was observed in rats, mice, hamsters, rabbits, dogs and primates; not in horses and sheep. In some cases these divergences were combined with defects of the central nervous system and of the heart. In primates, effects in the brain were seen after exposure. Moreover, intrauterine growth can be delayed. All these effects were seen at high dosages.
Effects in non-clinical studies were observed only at doses considered sufficiently in excess of the maximum dose for human indicating little relevance to clinical use.
No mutagenicity, carcinogenicity, reproductive or developmental toxicity data are available for OZURDEX. Dexamethasone has been shown to be teratogenic in mice and rabbits following topical ophthalmic application.
Dexamethasone exposure to the healthy/untreated eye via contralateral diffusion has been observed in rabbits following delivery of the implant to the posterior segment of the eye.
Repeat dose topical ocular safety studies with dexamethasone in rabbits have shown systemic corticosteroid effects. Such effects are considered to be unlikely when Onadron is used as recommended.
Dexamethasone was clastogenic in the in vitro human lymphocyte assay and in vivo in the mouse micronucleus assay at doses in excess of those obtained following topical application. Conventional carcinogenicity studies with Onadron have not been performed.
Dexamethasone has been found to be teratogenic in animal models. Dexamethasone induced abnormalities of foetal development including cleft palate, intra-uterine growth retardation and affects on brain growth and development.
There are no other preclinical data of relevance to the prescriber which are additional to that included in other sections of the SPC.
Incompatibilities
The information provided in Incompatibilities of Onadron
is based on data of another medicine with exactly the same composition as the Onadron.
. Be careful and be sure to specify the information on the section Incompatibilities in the instructions to the drug Onadron directly from the package or from the pharmacist at the pharmacy.
more…
None known.
Special precautions for disposal and other handling
The information provided in Special precautions for disposal and other handling of Onadron
is based on data of another medicine with exactly the same composition as the Onadron.
. Be careful and be sure to specify the information on the section Special precautions for disposal and other handling in the instructions to the drug Onadron directly from the package or from the pharmacist at the pharmacy.
more…
Eye drops; Eye suspension; Injection; Pills; Solution for intravenous and intramuscular injection; Substance-powder
Coated tablet; Intravitreal implant in applicator; Имплантат для интравитреального введения
Eye ointment
No special requirements for disposal.
OZURDEX is for single use only.
Each applicator can only be used for the treatment of a single eye.
If the seal of the foil pouch containing the applicator is damaged, the applicator must not be used. Once the foil pouch is opened the applicator should be used immediately.
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
Do not touch dropper tip to any surface as this may contaminate the contents.
If the drop of medication is not retained in the eye upon dosing for any reason then instill another drop.
Onadron price
We have no data on the cost of the drug.
However, we will provide data for each active ingredient
The approximate cost of Dexamethasone 4 mg per unit in online pharmacies is from 0.65$ to 1.53$, per package is from 39$ to 153$.
The approximate cost of Dexamethasone 0.5 mg per unit in online pharmacies is from 0.26$ to 0.76$, per package is from 21$ to 59$.
The approximate cost of Dexamethasone 0.5 mg/5ml per unit in online pharmacies is from 0.66$ to 0.88$, per package is from 88$ to 199$.
The approximate cost of Dexamethasone 0.5/5 mg/ml per unit in online pharmacies is from 0.53$ to 1.01$, per package is from 53$ to 101$.
The approximate cost of Dexamethasone 0.50 mg per unit in online pharmacies is from 0.49$ to 0.8$, per package is from 49$ to 80$.
The approximate cost of Dexamethasone 0.75 mg per unit in online pharmacies is from 0.68$ to 1.6$, per package is from 48$ to 124$.
The approximate cost of Dexamethasone 2 mg per unit in online pharmacies is from 0.55$ to 1.08$, per package is from 42$ to 108$.